Disease Management
Acromegaly isTreatable

Acromegaly is Treatable
The treatment goal is to bring hormone levels (e.g. IGF-1 and GH) back to normal level and relieve of symptoms.
Treatment options that is available in Malaysia includes:

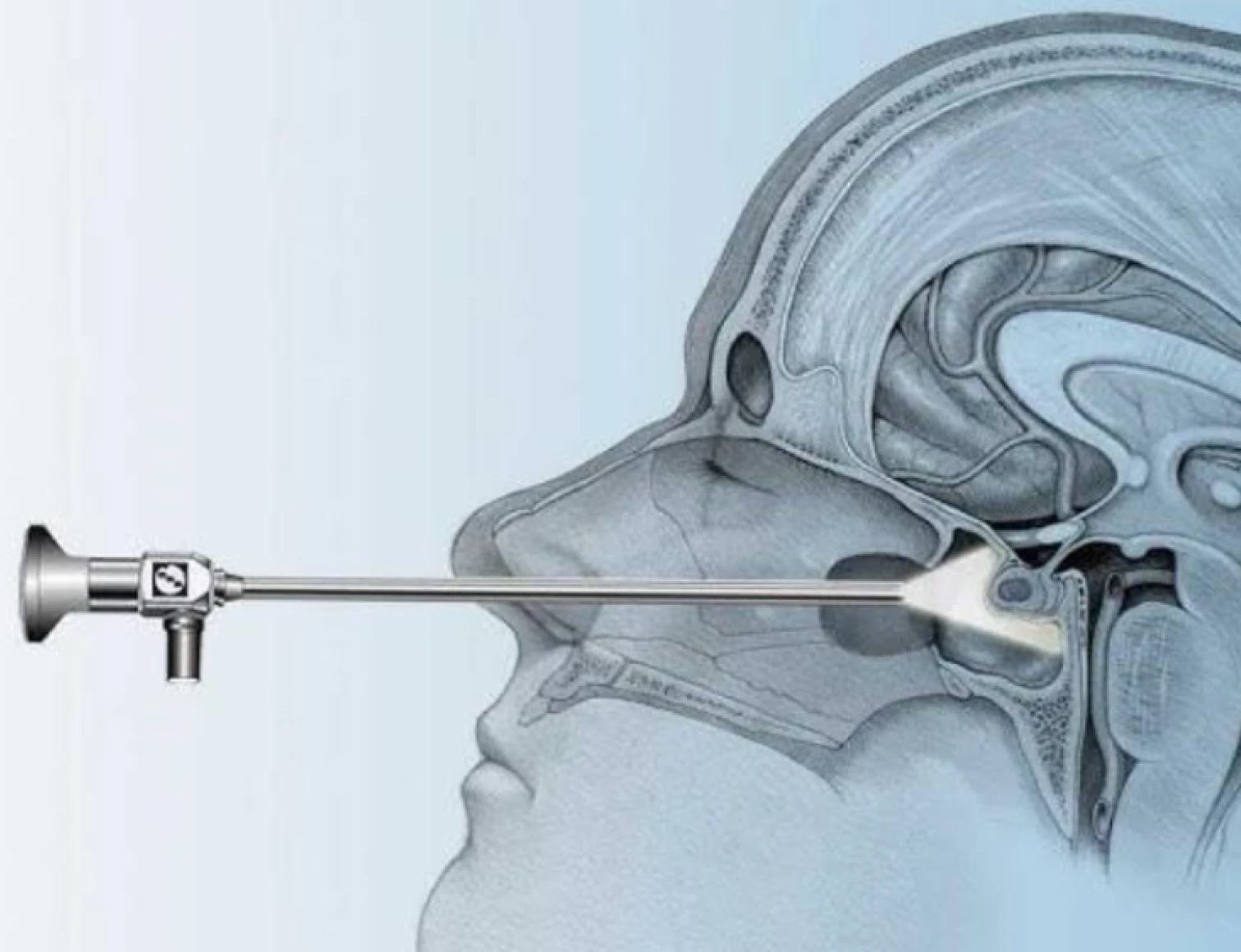
Transsphenoidal surgery is a minimally invasive brain operation that removes growths on the pituitary gland, by going through the nose.

Medical therapy for acromegaly is primarily used when surgery is not curative or is contraindicated. The main goals of treatment are to lower growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels, control tumor size, and manage related symptoms. The TWO main classes of drugs available in Malaysia used are:

SRLs are the most effective class of medical therapy for acromegaly and are often the first-line medical option. They act by binding to somatostatin receptors on the pituitary tumor, which suppresses GH secretion and can cause tumor shrinkage.

Individual who is unfit for or who refuse surgery with residual or recurrent tumours not amenable to repeat surgery Failed medical therapy
List of Malaysia Endocrine Centers
Wilayah Persekutuan
Selangor
Perak
Terengganu
Johor
Penang
Negeri Sembilan
Sarawak
Melaka
Sabah
Kedah
Kelantan
Pahang
WHAT YOU CAN DO
Doctor’s appointment preparations
Know what to do before your visit
When you make the appointment, ask if there's anything you need to do to prepare for testing.
Write down any symptoms you're having
Include any that seem unrelated to the appointment. For example, if you've had headaches more often or if you've been feeling down or more tired than usual, tell your health care provider. Also talk to your provider about changes in your appearance, such as weight gain, new acne or more body hair.
Write down key personal information
Let your provider know if the people closest to you have noticed that you seem irritable or have more mood swings than in the past. It may help to bring a photo of yourself that shows any changes in your appearance since you've started having symptoms.
Make a list of all medicines, vitamins, creams or supplements
Write down the name, dose and dates of any steroid medicines you've taken before, such as cortisone shots.
Take a family member or friend along, if possible
Sometimes it can be hard to remember all the information you get during an appointment. Someone who comes with you may remember something that you missed or forgot.
Write down questions
To ask your health care provider
